Posts Tagged ‘chelation’
Epic Ligand Survey: Dative Ligands of N, O, and S
 In this post, we’ll take a quick look at L-type ligands of nitrogen, oxygen, and sulfur. Ligands of this type are important for at least two reasons: (1) coordination to a metal can modify the reactivity of the bound functional group, and (2) dative coordination is a critical element of organometallic reactions that depend on intramolecular directing group effects. “Long-term” ligands containing two-connected nitrogens, such as pyridines and oxazolines, are now among the most commonly used for organometallic reactions. The behavior of coordinated dinitrogen is also a hot research area right now. Although they look boring on the surface, dative ligands of N, O, and S are rich in chemistry!
In this post, we’ll take a quick look at L-type ligands of nitrogen, oxygen, and sulfur. Ligands of this type are important for at least two reasons: (1) coordination to a metal can modify the reactivity of the bound functional group, and (2) dative coordination is a critical element of organometallic reactions that depend on intramolecular directing group effects. “Long-term” ligands containing two-connected nitrogens, such as pyridines and oxazolines, are now among the most commonly used for organometallic reactions. The behavior of coordinated dinitrogen is also a hot research area right now. Although they look boring on the surface, dative ligands of N, O, and S are rich in chemistry!
General Properties
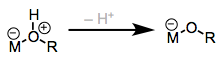
This might be the first class of ligand for which we can reliably say that backbonding is rarely important. Dative coordination of amines and alcohols involves a straightforward n → dσ orbital interaction. Intuitively, we should expect the acidity of amines, alcohols, and thiols to increase upon coordination, because removal of electron density from nitrogen and oxygen through coordination makes these atoms more electrophilic. Consider the charged model of dative bonding at left in the figure below.
Transfer of the lost proton to an organic substrate is an important aspect of hydrogenation reactions employing amine ligands (see below).
Food for thought: why aren’t (cheaper) amines found in place of phosphines in organometallic catalysts? History has ruled against tertiary amines, but are there any good reasons why? Yes—for one thing, amine nitrogens are more sterically hindered than analogous phosphorus atoms, because N–C bonds are shorter than P–C bonds. Plus, the cone angles of amines are generally wider than those of phosphines. Getting amines to play nice with hindered metal centers can thus be very difficult. Read the rest of this entry »
Epic Ligand Survey: Phosphines
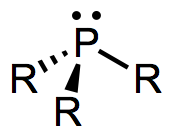 The epic ligand survey continues with tertiary phosphines, PR3. Phosphines are most notable for their remarkable electronic and steric tunability and their “innocence”—they tend to avoid participating directly in organometallic reactions, but have the ability to profoundly modulate the electronic properties of the metal center to which they’re bound. Furthermore, because the energy barrier to umbrella flipping of phosphines is quite high, “chiral-at-phosphorus” ligands can be isolated in enantioenriched form and introduced to metal centers, bringing asymmetry just about as close to the metal as it can get in chiral complexes. Phosphorus NMR is a technique that Just Works (thanks, nature). Soft phosphines match up very well with the soft low-valent transition metals. Electron-poor phosphines are even good π-acids! Need I say more? Let’s explore this fascinating, ubiquitous class of ligands in more detail.
The epic ligand survey continues with tertiary phosphines, PR3. Phosphines are most notable for their remarkable electronic and steric tunability and their “innocence”—they tend to avoid participating directly in organometallic reactions, but have the ability to profoundly modulate the electronic properties of the metal center to which they’re bound. Furthermore, because the energy barrier to umbrella flipping of phosphines is quite high, “chiral-at-phosphorus” ligands can be isolated in enantioenriched form and introduced to metal centers, bringing asymmetry just about as close to the metal as it can get in chiral complexes. Phosphorus NMR is a technique that Just Works (thanks, nature). Soft phosphines match up very well with the soft low-valent transition metals. Electron-poor phosphines are even good π-acids! Need I say more? Let’s explore this fascinating, ubiquitous class of ligands in more detail.
General Properties
Like CO, phosphines are dative, L-type ligands that formally contribute two electrons to the metal center. Unlike CO, most phosphines are not small enough to form more than four bonds to a single metal center (and for large R, the number is even smaller). Steric hindrance becomes a problem when five or more PR3 ligands try to make their way into the space around the metal. An interesting consequence of this fact is that many phosphine-containing complexes do not possess 18 valence electrons. Examples include Pt(PCy3)2, Pd[P(t-Bu)3]2, and [Rh(PPh3)3]+. Doesn’t that just drive you crazy? It drives the complexes crazy as well—and most of these coordinatively unsaturated compounds are wonderful catalysts.
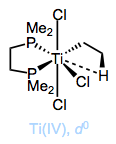
Bridging by phosphines is extremely rare, but ligands containing multiple phosphine donors that bind in an Ln (n > 1) fashion to a single metal center are all over the place. These ligands are called chelating or polydentate to indicate that they latch on to metal centers through multiple binding sites. For entropic reasons, chelating ligands bind to a single metal center at multiple points if possible, instead of attaching to two different metal centers (the aptly named chelate effect). An important characteristic of chelating phosphines is bite angle, defined as the predominant P–M–P angle in known complexes of the ligand. We’ll get into the interesting effects of bite angle later, but for now, we might imagine how “unhappy” a ligand with a preferred bite angle of 120° would be in the square planar geometry. It would much rather prefer to be part of a trigonal bipyramidal complex, for instance.
Read the rest of this entry »