Posts Tagged ‘structure’
Ligand Field Theory & Frontier Molecular Orbital Theory
In this post, we’ll begin to explore the molecular orbital theory of organometallic complexes. Some background in molecular orbital theory will be beneficial; an understanding of organic frontier molecular orbital theory is particularly helpful. Check out Fukui’s Nobel Prize lecture for an introduction to FMO theory. The theories described here try to address how the approach of ligands to a transition metal center modifies the electronics of the metal and ligands. The last post on geometry touched on these ideas a little, but we’ll dig a little deeper here. Notably, we need to address the often forgotten influence of the metal on the ligands—how might a metal modify the reactivity of organic ligands?
Ligand Field Theory
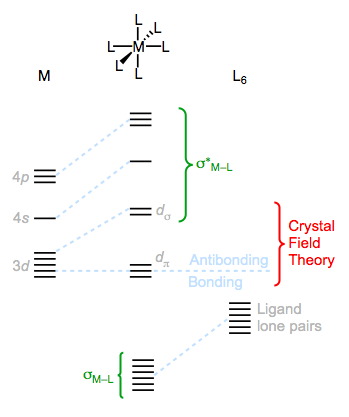
The ligand field theory (LFT) fleshes out the ideas of crystal field theory with molecular orbital theory concepts. It provides a method for understanding M–L bonding and antibonding orbitals; however, it has been strongly disputed by computational studies in favor of valence bond models that incorporate hypervalency. Still, LFT provides a more complete picture of complex bonding than crystal field theory, so we’ll discuss it here. Furthermore, the portions of LFT under dispute have nothing to do with CFT, so “no harm no foul.” Let’s take a look at the molecular orbitals of a hypothetical octahedral MLn complex to begin hashing out LFT.
Simplifying the Organometallic Complex (Part 3)
So far, we’ve seen how deconstruction can reveal useful “bookkeeping” properties of organometallic complexes: number of electrons donated by ligands, coordination number, oxidation state, and d electron count (to name a few). Now, let’s bring everything together and discuss total electron count, the sum of non-bonding and bonding electrons associated with the metal center. Like oxidation state, total electron count can reveal the likely reactivity of OM complexes—in fact, it is often more powerful than oxidation state for making predictions. We’ll see that there is a definite norm for total electron count, and when a complex deviates from that norm, reactions are likely to happen.
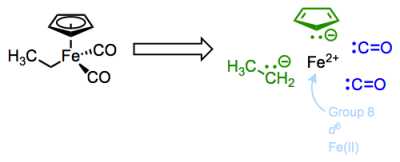
Let’s begin with yet another new complex. This molecule features the common and important cyclopentadienyl and carbon monoxide ligands, along with an X-type ethyl ligand.
The Cp or cyclopentadienyl ligand is a polydentate, six-electron L2X ligand. The two pi bonds of the free anion are dative, L-type ligands, which we’ll see again in a future post on ligands bound through pi bonds. Think of the electrons of the pi bond as the source of a dative bond to the metal. Since both electrons come from the ligand, the pi bonds are L-type binders. The anionic carbon in Cp is a fairly standard, anionic X-type binder. The carbon monoxide ligands are interesting examples of two-electron L-type ligands—notice that the free ligands are neutral, so these are considered L-type! Carbon monoxide is an intriguing ligand that can teach us a great deal about metal-ligand bonding in OM complexes…but more on that later. Read the rest of this entry »
Simplifying the Organometallic Complex (Part 1)
Organometallic complexes, which consist of centrally located metals and peripheral organic compounds called ligands, are the workhorses of organometallic chemistry. Just like organic intermediates, understanding something about the structure of these molecules tells us a great deal about their expected reactivity. Some we would expect to be stable, and others definitely not! A big part of our early explorations will involve describing, systematically, the principles that govern the stability of organometallic complexes. From the outset, I will say that these principles are not set in stone and are best applied to well controlled comparisons. Nonetheless the principles are definitely worth talking about, because they form the foundation of everything else we’ll discuss. Let’s begin by exploring the general characteristics of organometallic complexes and identifying three key classes of organic ligands.
When we think of metals we usually think of electropositive atoms or even positively charged ions, and many of the metals of OM chemistry fit this mold. In general, it is useful to imagine organic ligands as electron donors and metals as electron acceptors. When looking at a pair of electrons shared between a transition metal and main-group atom (or hydrogen), I imagine the cationic metal center and anionic main-group atom racing toward one another from oblivion like star-crossed lovers. In the opposite direction (with an important caveat that we’ll address soon), we can imagine ripping apart metal–R covalent bonds and giving both electrons of the bond to the organic atom. This heterolytic bond cleavage method reproduces the starting charges on the metal and ligand. Unsurprisingly, the metal is positive and the ligand negative.