Posts Tagged ‘beta-hydride elimination’
β-Elimination Reactions
In organic chemistry class, one learns that elimination reactions involve the cleavage of a σ bond and formation of a π bond. A nucleophilic pair of electrons (either from another bond or a lone pair) heads into a new π bond as a leaving group departs. This process is called β-elimination because the bond β to the nucleophilic pair of electrons breaks. Transition metal complexes can participate in their own version of β-elimination, and metal alkyl complexes famously do so. Almost by definition, metal alkyls contain a nucleophilic bond—the M–C bond! This bond can be so polarized toward carbon, in fact, that it can promote the elimination of some of the world’s worst leaving groups, like –H and –CH3. Unlike the organic case, however, the leaving group is not lost completely in organometallic β-eliminations. As the metal donates electrons, it receives electrons from the departing leaving group. When the reaction is complete, the metal has picked up a new π-bound ligand and exchanged one X-type ligand for another.

Comparing organic and organometallic β-eliminations. A nucleophilic bond or lone pair promotes loss or migration of a leaving group.
In this post, we’ll flesh out the mechanism of β-elimination reactions by looking at the conditions required for their occurrence and their reactivity trends. Many of the trends associated with β-eliminations are the opposite of analogous trends in 1,2-insertion reactions. A future post will address other types of elimination reactions.
β-Hydride Elimination
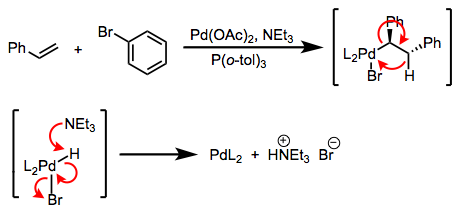
The most famous and ubiquitous type of β-elimination is β-hydride elimination, which involves the formation of a π bond and an M–H bond. Metal alkyls that contain β-hydrogens experience rapid elimination of these hydrogens, provided a few other conditions are met. Read the rest of this entry »
Migratory Insertion: 1,2-Insertions
Insertions of π systems into M-X bonds are appealing in the sense that they establish two new σ bonds in one step, in a stereocontrolled manner. As we saw in the last post, however, we should take care to distinguish these fully intramolecular migratory insertions from intermolecular attack of a nucleophile or electrophile on a coordinated π-system ligand. The reverse reaction of migratory insertion, β-elimination, is not the same as the reverse of nucleophilic or electrophilic attack on a coordinated π system.
Like 1,1-insertions, 1,2-insertions generate a vacant site on the metal, which is usually filled by external ligand. For unsymmetrical alkenes, it’s important to think about site selectivity: which atom of the alkene will end up bound to metal, and which to the other ligand? To make predictions about site selectivity we can appeal to the classic picture of the M–X bond as M+X–. Asymmetric, polarized π ligands contain one atom with excess partial charge; this atom hooks up with the complementary atom in the M–R bond during insertion. Resonance is our best friend here!
A nice study by Yu and Spencer illustrates these effects in homogeneous palladium- and rhodium-catalyzed hydrogenation reactions. Unactivated alkenes generally exhibit lower site selectivity than activated ones, although steric differences between the two ends of the double bond can promote selectivity. Read the rest of this entry »
Epic Ligand Survey: Metal Alkyls (Part 1)
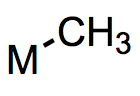 With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands, although some alkyl ligands behave as spectators. Our aim will be to understand the general dependence of the behavior of alkyl ligands on the metal center and the ligand’s substituents. Using this knowledge, we can make meaningful comparisons between related metal alkyl complexes and educated predictions about their likely behavior. Because alkyl ligands are central to organometallic chemistry, I’ve decided to spread this discussion across multiple posts. We’ll deal first with the general properties of metal alkyls.
With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands, although some alkyl ligands behave as spectators. Our aim will be to understand the general dependence of the behavior of alkyl ligands on the metal center and the ligand’s substituents. Using this knowledge, we can make meaningful comparisons between related metal alkyl complexes and educated predictions about their likely behavior. Because alkyl ligands are central to organometallic chemistry, I’ve decided to spread this discussion across multiple posts. We’ll deal first with the general properties of metal alkyls.
General Properties
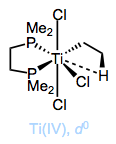
In the Simplifying the Organometallic Complex series, we decomposed the M–C bond into a positively charged metal and negatively charged carbon. This deconstruction procedure is consistent with the relative electronegativities of carbon and the transition metals. It can be very useful for us to imagine metal alkyls essentially as stabilized carbanions—but it’s also important to understand that M–C bonds run the gamut from extremely ionic and salt-like (NaCH3) to essentially covalent ([HgCH3]+). The reactivity of the alkyl ligand is inversely related to the electronegativity of the metal center.
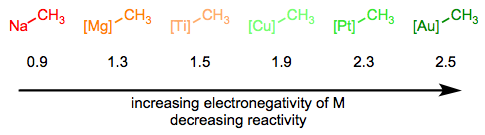
Reactivity decreases as the metal's electronegativity increases. Values given are Pauling electronegativities.
The hybridization of the carbon atom is also important, and the trend here follows the trend in nucleophilicity as a function of hybridization in organic chemistry. sp-Hybridized ligands are the least nucleophilic, followed by sp2 and sp3 ligands respectively. Read the rest of this entry »