Posts Tagged ‘thermodynamic cycles’
Epic Ligand Survey: Metal Hydrides
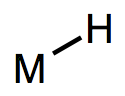 Metal-hydrogen bonds, also known (misleadingly) as metal hydrides, are ubiquitous X-type ligands in organometallic chemistry. There is much more than meets the eye to most M-H bonds: although they’re simple to draw, they vary enormously in polarization and pKa. They may be acidic or hydridic or both, depending on the nature of the metal center and the reaction conditions. In this post, we’ll develop some heuristics for predicting the behavior of M-H bonds and discuss their major modes of reactivity (acidity, radical reactions, migratory insertion, etc.). We’ll also touch on the most widely used synthetic methods to form metal hydrides.
Metal-hydrogen bonds, also known (misleadingly) as metal hydrides, are ubiquitous X-type ligands in organometallic chemistry. There is much more than meets the eye to most M-H bonds: although they’re simple to draw, they vary enormously in polarization and pKa. They may be acidic or hydridic or both, depending on the nature of the metal center and the reaction conditions. In this post, we’ll develop some heuristics for predicting the behavior of M-H bonds and discuss their major modes of reactivity (acidity, radical reactions, migratory insertion, etc.). We’ll also touch on the most widely used synthetic methods to form metal hydrides.
General Properties
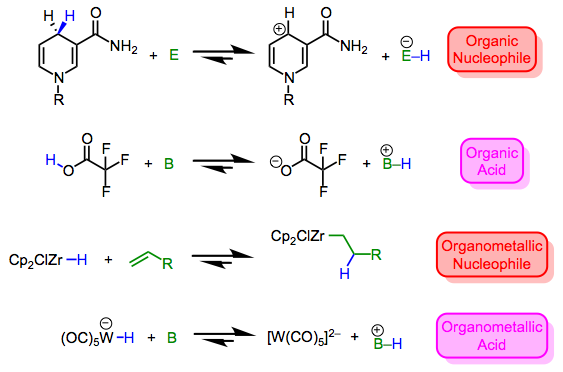
Metal hydrides run the gamut from nucleophilic/basic to electrophilic/acidic. Then again, the same can be said of X–H bonds in organic chemistry, which may vary from mildly nucleophilic (consider Hantzsch esters and NADH) to extremely electrophilic (consider triflic acid). As hydrogen is what it is in both cases, it’s clear that the nature of the X fragment—more specifically, the stability of the charged fragments X+ and X–—dictate the character of the X–H bond. Compare the four equilibria outlined below—the stabilities of the ions dictate the position of each equilibrium. By now we shouldn’t find it surprising that the highly π-acidic W(CO)5 fragment is good at stabilizing negative charge; for a similar reason, the ZrCp2Cl fragment can stabilize positive charge.*